
Excipient, ICH-Q7 GMP Manufactured Product
Guanidine HCL, NF, LBLE*, GMP, Excipient Grade
Product Code: GHCL-3253
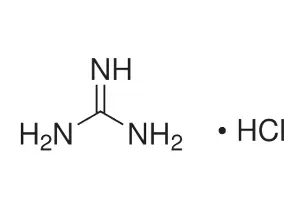
CAS #: 50-01-1
Formula: CH5N3 • HCl
Sol. In H2O (g/L): 573
F.W.: 95.53 g/mol
pKa @ 20˚C: 4.5 – 6.0
Minimum Order Quantity: 500kg | Lead Time (if no stock): 1-month | Package Sizes: 10kg, 25 kg and 50 kg pails.
Intended For Critical Biopharma Applications
General Product Description:
GMP Compliance:
Retest Date:
Storage and Shipping Conditions:
Package Sizes: