
Excipient, ICH-Q7 GMP Manufactured Product
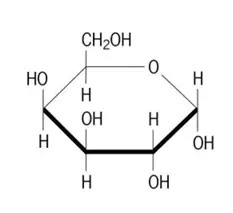
Galactose(D), Plant Derived, EP, NF, LBLE*, GMP Grade
*Low Bioburden, Low Endotoxin, **Low Elemental Impurities
Product Code: GALP-3350
CAS #: 59-23-4
Formula: C6H12O6
Sol. In H2O (g/L): 600
F.W.: 180.16 g/mol
pKa @ 25˚C: 12.4
Minimum Order Quantity: 25kg | Lead Time: If Stock, 1-month/ No Stock, 6-months | Package Sizes: 10kg & 25kg pails
Intended for Use in Pharma Mfg. Requiring ICH-Q7, Excipient Grade Quality & Regulatory Compliance
General Product Description:
GMP Compliance:
Retest Date:
Storage and Shipping Conditions:
Package Sizes: