
Excipient / ICH-Q7 GMP Manufactured Product
Urea, USP, EP, LBLE*, GMP, Excipient Grade
*Low Bioburden, Low Endotoxin
Product Code: UREA-3250
CAS #: 57-13-6
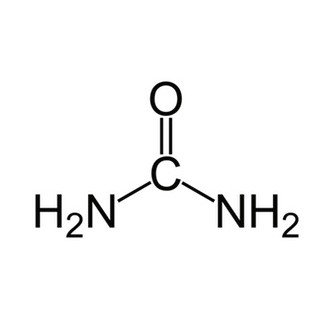
Formula: CH4N2O
Sol. In H2O (g/L): 480 @ 20˚C
F.W.: 60.06 g/mol
pH @ 20°C: 7.2 (10% soln.)
Minimum Order Quantity: 500kg | Lead Time (if no stock): 3 months | Package Sizes: 10kg, 25kg and 50kg pails.
Intended For Use As An Excipient
General Product Description:
Product Statements:
GMP Compliance:
Retest Date:
Storage and Shipping Conditions:
Package Sizes: