
GMP Solution / ICH-Q7 GMP Manufactured Product
Urea (6M) Solution, GMP, Excipient Grade
*Low Bioburden, Low Endotoxin
Product Code: UREA-3120
CAS #: 57-13-6
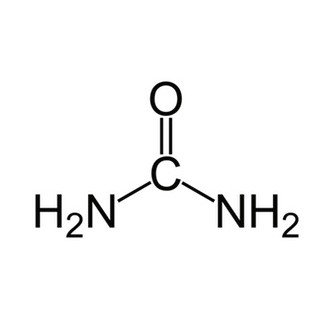
Formula: CH4N2O
360 grams per Liter Urea
pH of a 6M soln., 25°C = 7-10
Minimum Order Quantity: 4,000-liters | Lead Time (if no stock): 3-months | Package Sizes: 200 Liter drums, 1,000 – 1,200 L totes.
Intended For Use As An Excipient Grade Solution
General Product Description:
The manufacturing of Bio Excipient Grade Urea 6M soln.,
UREA-3120 is performed at BioSpectra’s Bangor, PA facility and is conducted in a multi-purpose processing area using multi-purpose equipment.
GMP Compliance:
Retest Date:
Storage and Shipping Conditions:
Package Sizes: