
Buffer / Excipient / ICH-Q7 GMP Manufactured Product
Tris Excipient, USP, EP, JP, LBLE*, GMP, Excipient Grade
*Low Bioburden, Low Endotoxin
Product Code: TRIS-3255
CAS #: 77-86-1
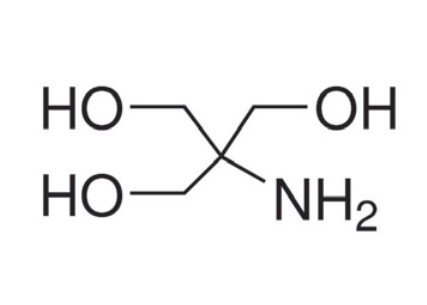
Formula: C4H11NO3
Sol. In H2O @ 25°C (g/L): 550
F.W.: 121.14 g/mol
pH @ 20°C 5% aq.: 10.0 – 11.5
Useful pH: 7.0 – 9.0
pKa @ 20˚C: 8.3
Minimum Order Quantity: 500kg | Lead Time (if no stock): 3-months | Package Sizes: 10kg, 25kg and 50kg pails.
Intended For Use As An Excipient
General Product Description:
Product Statements:
Metal Residue Statement: BioSpectra certifies that TRIS BASE, Product Code TRIS-3255, manufactured by BioSpectra is manufactured without the use of metal catalysts and metal reagents. There are no known risks to metal residues in this product.
Melamine Statement: Based on the manufacturing process and the controlled handling, storage, and analysis of Tris, Product Code
TRIS-3255, BioSpectra certifies that there is low risk for melamine contamination. Additionally, when tested for melamine, this product reports less than 0.15 ppm.
Residual Solvents Statement: Based on the manufacturing process and the controlled handling, storage and analysis of this product, this product complies with the requirements and specifications listed in the current USP method <467> Tables 1, 2, 3, or 4.
GMP Compliance:
Expiration:
Storage and Shipping Conditions:
Package Sizes: